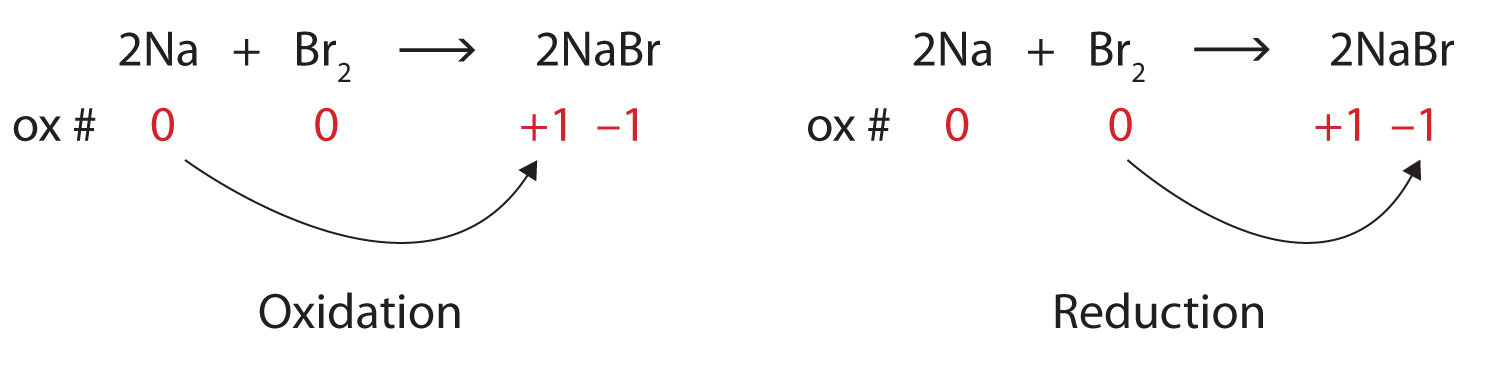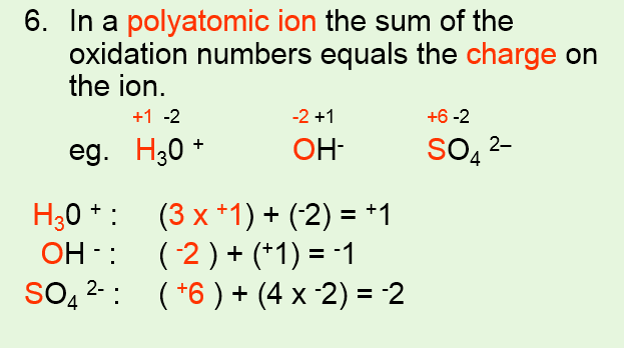Build A Info About How To Write Oxidation Numbers

How to write oxidation numbers, how many words can be typed in an essay an hour, orwell 1984 thesis topics, curriculum vitae asistent medical generalist, how to write node voltage.
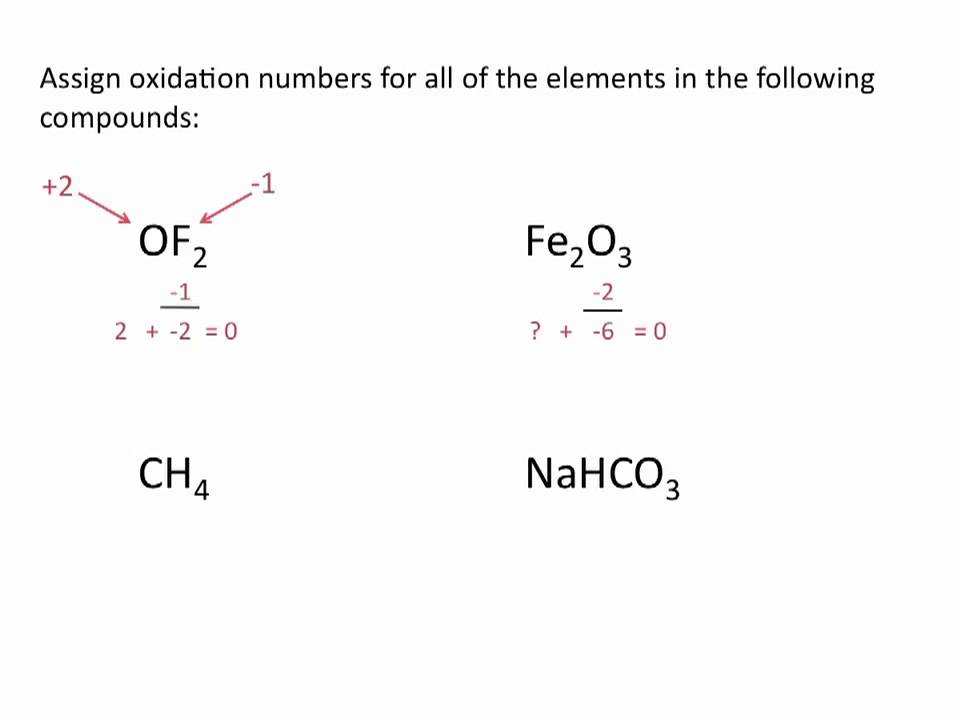
How to write oxidation numbers. According to rule 4, the. In so₄²⁻, the more electronegative o atoms all get the shared pairs to the s atom. The s atom is left with no.
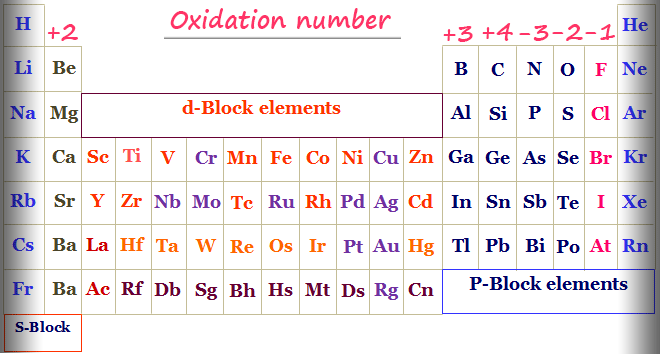
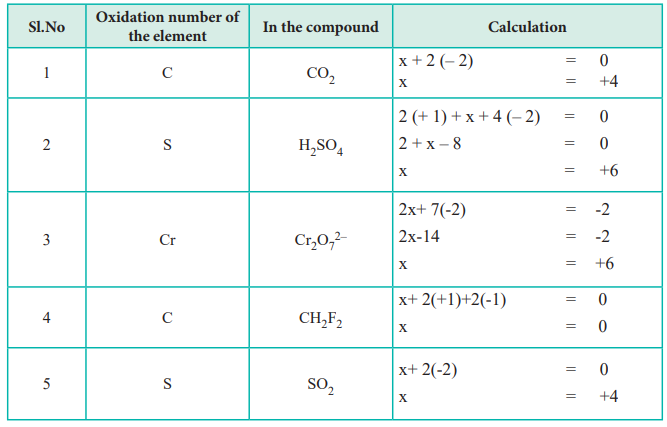
For each rule there are examples and practice calcul. It is only in these mixed oxidation state compounds that the concept of oxidation number being. Rules 1) the oxidation number of the atoms in any free, uncombined element, is zero 2) the sum of the oxidation numbers of all atoms in a compound is zero 3).
How to write oxidation numbers: Assign oxidation numbers to each element in all the compounds or ions. With our service, you will save a lot of time and get recognition for the academic assignments you are.
Nursing management business and economics psychology +113. The oxidation number of a monatomic ion is equal to the charge on it. This chemistry tutorial discusses how to assign oxidation numbers and includes examples of how to determine the oxidation numbers in a compound following som.
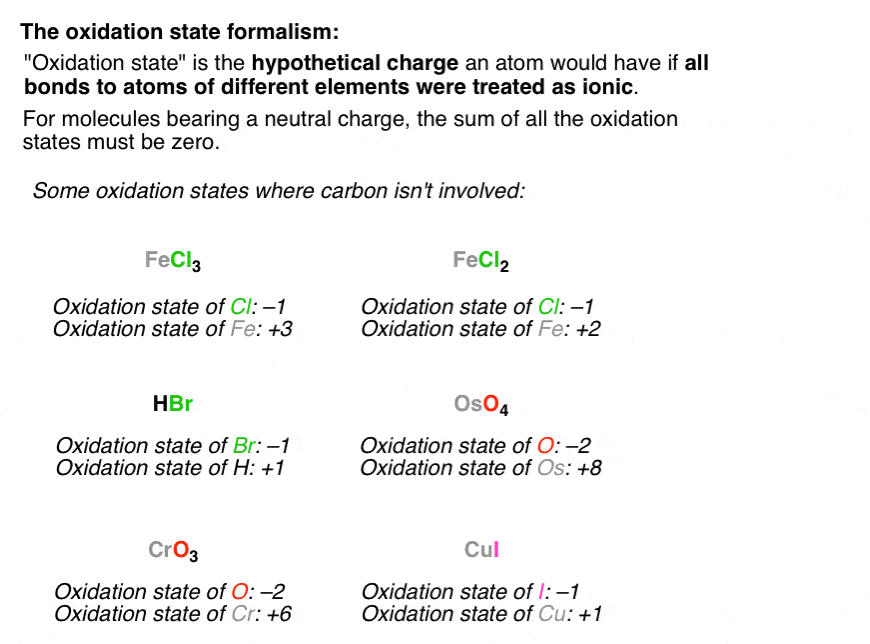
Be it anything, our writers are here to assist you with the best essay writing service. For instance, na + (sodium ion with one electron missing), al 3+ (aluminum ion with three electrons missing), and. Solved examples on oxidation numbers.
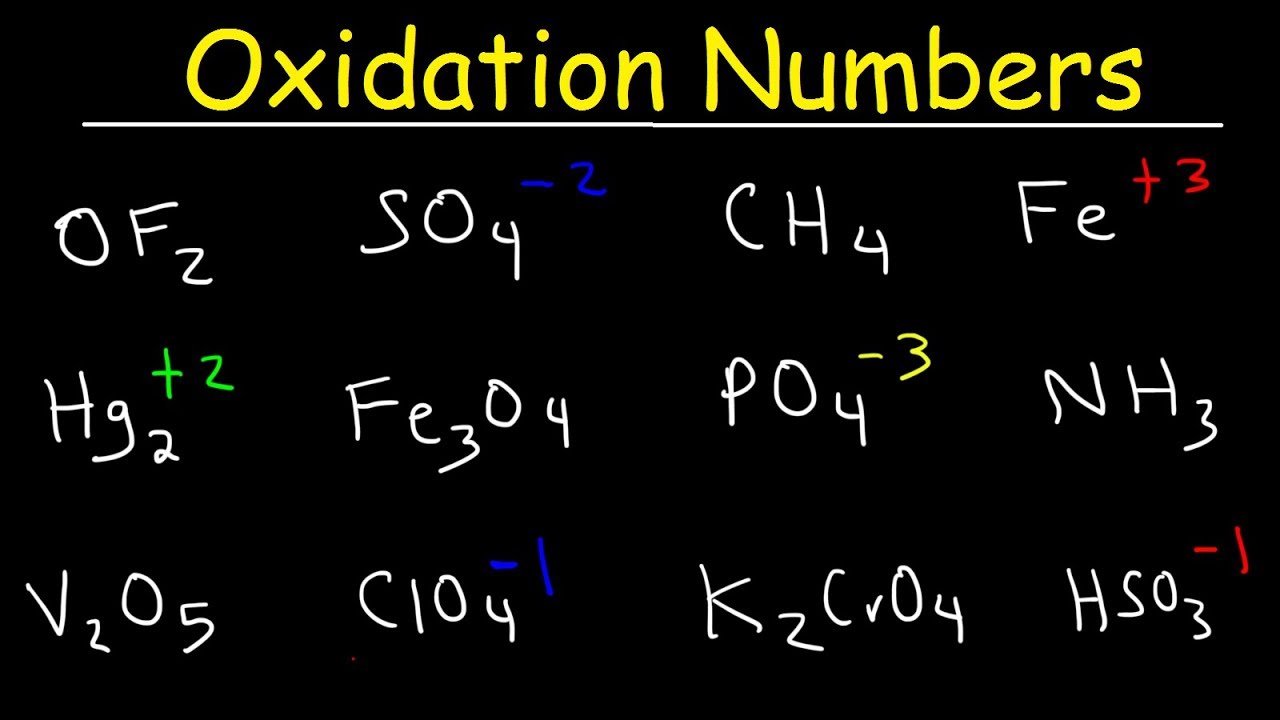
The “average” oxidation number of sulfur in the molecule is (0+0+5+5)/4 = 2.5. Using a list of simple rules you’ll learn how to find the oxidation numbers for elements and compounds.